RAPEX NETWORK
Based on the General Product Safety Directive (Directive 2001/95/EC – GPSD), there is a rapid alert system (RAPEX) for dangerous non-food products in the European Union (EU) since 2005. Through this system, national authorities (responsible for product safety) are able to send alerts to the European Commission, reporting the measures taken against products posing a risk. Companies and consumers may report dangerous products to the national authorities, through the Product Safety Business Alert Gateway.
Every week, the RAPEX system publishes notifications of urgent measures taken by EU Member-States to prevent, restrict or impose conditions on the marketing of products due to the serious and immediate danger they pose to the health and safety of consumers.
March this year, EU’s Safety Gate European RAPEX 2020 annual report was published.
2020 RAPEX REPORT
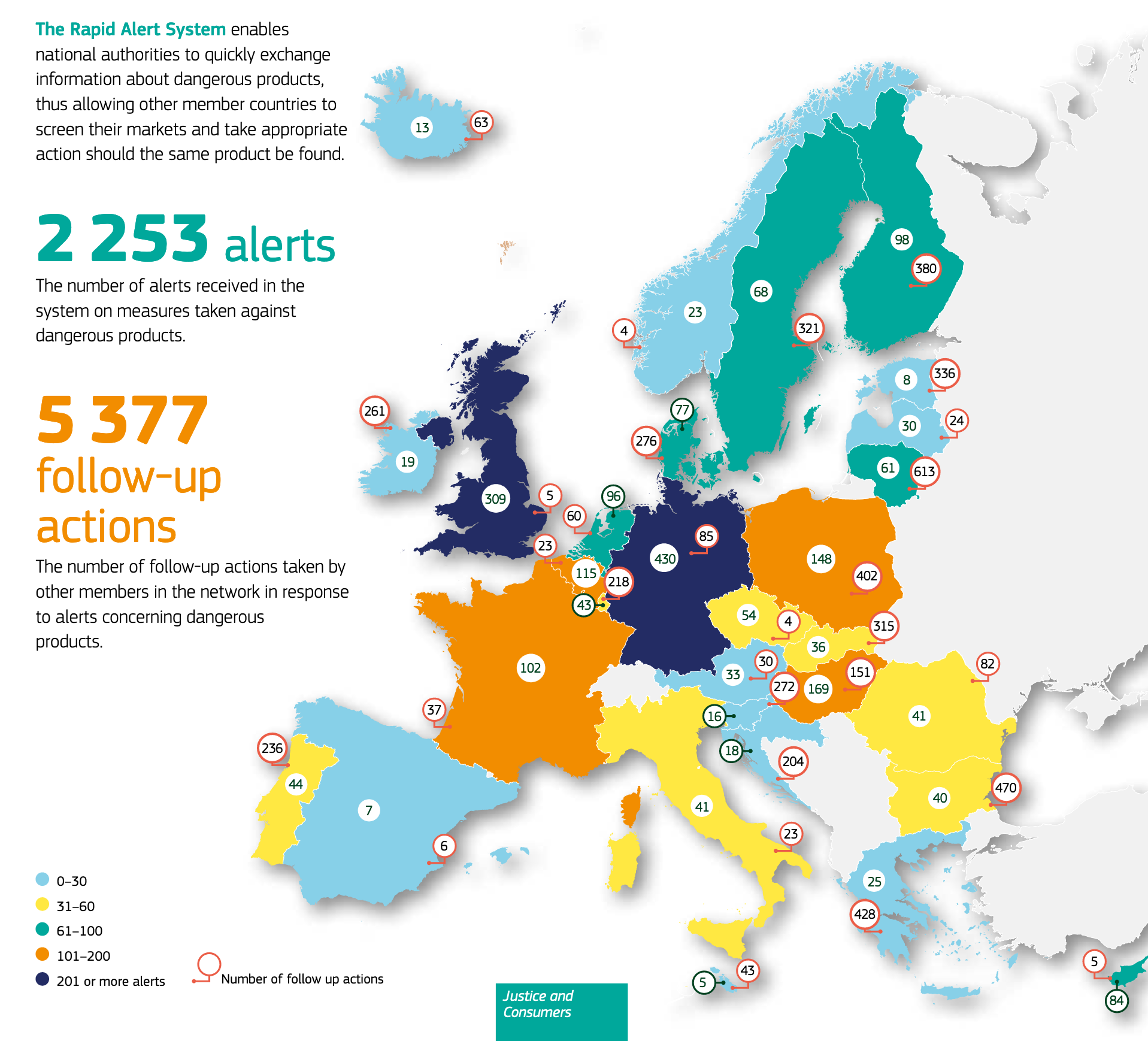
In 2020, there were 2,253 alerts received in the system on measures taken against dangerous products. There were 5,377 follow-up actions taken by other members in response to alerts concerning dangerous products.
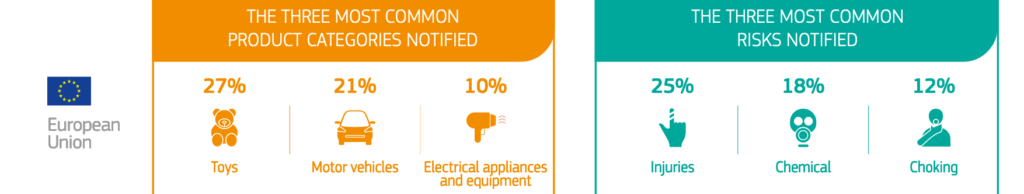
In the EU, the three most common product categories notified were toys (27%), motor vehicles (21%) and electrical appliances and equipment (10%). The three most common risks notified were injuries (25%), chemical (18%) and choking (12%).
In Ireland, Czech Republic, Greece, Spain, Austria and Sweden, cosmetics were among these 3 most common product categories notified, representing 42%, 20%, 4%, 43%, 15% and 10%, respectively. Overall, in the EU there were a total of 86 alerts related with cosmetics, 16 were notified by Germany and 11 by the Czech Republic, representing the two countries with more alert notifications. 67 notifications were related with chemical risks, 12 with microbiological risk, 9 related with other health risks and one with choking. Many cosmetics notified came originally from countries outside the EU, like China (14 alerts) and Pakistan (12 alerts).
In total there were 263 follow-ups on cosmetics, 236 related with chemical risk. Lithuania represented the country with more follow-ups (37), followed by Poland (25) and Bulgaria (24).
For example, in Czech Republic, 20% alerts on cosmetics amounted to 11 notifications. One of the main reasons for notification was the detection of substances with demonstrable allergenic potential which were not included in the ingredients list.
COVID-19 PANDEMIC RELATED PRODUCTS AND ONLINE PRODUCTS
In 2020, the Safety Gate also reflected on the impact of the COVID-19 pandemic. Due to COVID-19, more products (e.g. masks, hand disinfectants) became available to consumers and and regularly included in their shopping list. Some of these products did not meet the EU health and safety requirements, giving a false sense of protection to consumers. Masks, UV sterilizers, coveralls and hand sanitizers represented the highest number of alerts reported by the national authorities.
In order to support national authorities, the European Commission launched a coordinated activity with national authorities to tackle dangerous COVID-19 related products. Its objectives are to organize market surveillance activities on COVID-19 related dangerous products, find common evaluation practices for these products and the right communication tools to inform consumers. The results will be published by the Commission in 2021 on the Safety Gate website.
Alerts on products sold online were significantly higher (26%) when compared with the previous year (16%). This represents a big concern, mainly because checking and testing these products can be difficult, especially if the seller is located in a third country.
In 2021, the EU will continue working with the national authorities to improve product safety, particularly focusing in three areas of concern: COVID-19, online and recalls. On the other hand, the GPSD revision will continue with the aim of guaranteeing product safety in the future.
The EU Product Safety Award, which “rewards businesses that go the extra mile to protect consumers”, will have two award categories this year: protecting the safety of vulnerable consumer groups and combining safety and new technologies.
References:
- Safety Gate – 2020 results. Available from: https://www.dvsi.de/images/Safety_Gate_2020_Results.pdf
- EU Rapid Alert System factsheets 2020. Available from: https://ec.europa.eu/safety/consumers/consumers_safety_gate/statisticsAndAnualReports/2020/RAPEX_2020_Factsheet_EN.pdf
- Safety Gate: the EU rapid alert system for dangerous non-food products. Product Safety Award. Available from: https://ec.europa.eu/safety-gate/#/screen/pages/safetyAward