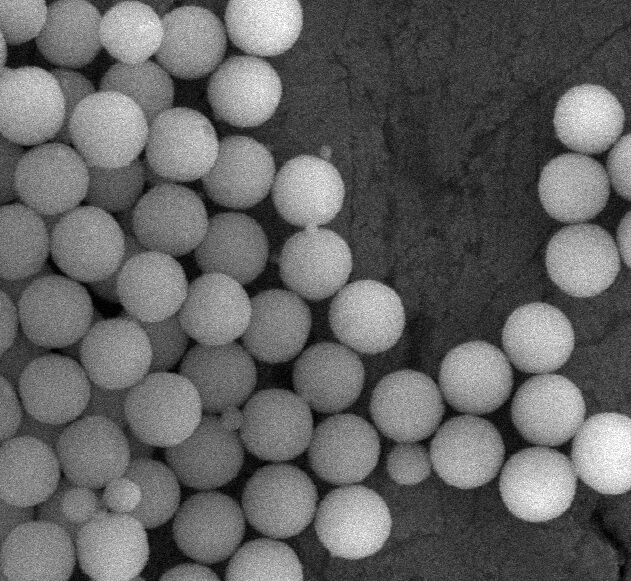
Silica, also known as silicone dioxide, is a mineral composed of silicon and oxygen and is used in cosmetics and personal care products, especially in make-up products. The type of Silica used in these products is the amorphous silica, which means it does not have a specific arrangement of the atoms and consequently it does not have a definite form. Silica (amorphous form) is also used as a food additive in the European Union (EU) and United States (U.S. approved by the Food and Drug Administration (FDA)). Silica related products, such as hydrated silica, alumina magnesium metasilicate, aluminium calcium sodium silicate, aluminium iron silicates and sodium potassium aluminium silicate, are also used as cosmetic ingredients in the EU.
According to the European Cosmetic Regulation No. 1223/2009, a ‘nanomaterial’ is described as “an insoluble or biopersistant and intentionally manufactured material with one or more external dimensions, or an internal structure, on the scale from 1 to 100 nm”. Cosmetic products containing ‘nanomaterials’ are subject to a specific notification to the European Commission and the Responsible Person must notify “it by electronic means six months prior to being placed on the market”.
The European Commission’s Scientific Committee on Consumer Safety (SCCS) has issued an opinion regarding Silica (Synthetic Amorphous Silica – SAS) and its solubility and consequent classification as a nanomaterial. The SCCS stated that “In regard to the nanomaterial definition in the Cosmetic Regulation, none of the SAS materials (hydrophilic or hydrophobic) included in the dossier can be regarded as soluble”. It means that the SCCS considered that Silica is insoluble and, if more than 50% of its particles has a size below 100 nanometers, it may classify as a nanomaterial. This opinion applies to Silica and related materials (Hydrated Silica, Silica Dimethyl Silylate, Silica Silylate, Silica Dimethicone Silylate, Silica Caprylyl Silylate and, Silica Cetyl Silylate).
The Association of Synthetic Amorphous Silica Producers (ASASP) is composed by 9 members that represent the majority of synthetic amorphous silica (SAS) producers in Europe and considers that SAS is soluble and should not be regarded as a nanomaterial.
Nevertheless, the suppliers of raw materials containing Silica must provide documentation regarding the particle size distribution in the ingredient to confirm if the particle size is below the cut-off limit and if it consequently needs to be regarded as the nano-form (according to SCCS opinion).
If a cosmetic product contains Silica in the nano-form (or other ingredient in this form), it must be clearly indicated in the list of ingredients in the product label. The name of the ingredient shall be followed by the word ‘nano’ in brackets (e.g. Silica [NANO]).
The SCCS has published a scientific advice on the safety of nanomaterials in cosmetics. The Committee has identified some aspects of nanomaterials that constitute a basis for concern over safety to consumers’ health when used in cosmetics, including physicochemical aspects (e.g. very small dimensions of particles), exposure aspects (e.g. frequency and amounts applied, potential for systemic exposure and accumulation in the body) and other aspects (like novel properties). These concerns where detailed in the document in three separate annexes.
With the development of scientific knowledge and continuous investigation, nanomaterials are being used in cosmetics more and more. Nevertheless, caution must be taken since there are certain aspects of these ingredients that are not yet fully known and may represent a safety concern.
References:
- European Food Safety Authority – Re-evaluation of Silicon Dioxide (E 551) as a food additive. Available at: https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2018.5088
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Available at: https://ec.europa.eu/health/sites/health/files/endocrine_disruptors/docs/cosmetic_1223_2009_regulation_en.pdf
- Scientific Committee on Consumer Safety (SCCS). Opinion on solubility of Synthetic Amorphous Silica (SAS). SCCS/1606/19. Available at: https://ec.europa.eu/health/sites/health/files/scientific_committees/consumer_safety/docs/sccs_o_228.pdf
- Synthetic Amorphous Silica (SAS) industry response to the opinion of the Scientific Committee on Consumer Safety (SCCS) on silica in cosmetics with regard to solubility. Available at: https://www.asasp.eu/images/Publications/ASASP1101b_-_ASASP_response_to_solubility_SCCS_Opinion_Dec_2019_final.pdf
- Scientific Committee on Consumer Safety (SCCS). Scientific Advice on the safety of nanomaterials in cosmetics (SCCS/1618/20). Available at: https://ec.europa.eu/health/sites/health/files/scientific_committees/consumer_safety/docs/sccs_o_239.pdf