BENZOPHENONE-3 AND OCTOCRYLENE
Benzophenone-3 (CAS number 131-57-7) and Octocrylene (CAS number 6197-30-4) are commonly used UV filters in cosmetics products. They are both included in Annex VI (list of UV filters allowed in cosmetic products) of the European Cosmetics Regulation (No. 1223/2009).
In early 2019, a priority list of 28 potential endocrine disruptors (not already covered by the bans of cosmetic regulation) was established by the European Commission. From these 28 substances, 14 were considered as higher priority (Group A) and the other 14 were included in the low priority group (Group B). The public call for data for the considered higher priority substances was carried out in 2019. (see previous post)
Stakeholders submitted scientific evidence to demonstrate the safety of Benzophenone-3 and Octocrylene as UV filters in cosmetic products and the European Commission asked the Scientific Committee for Consumer Safety (SCCS) to carry out a safety assessment of these ingredients, considering the information provided.
In March 2021 the SCCS published its opinions on Benzophenone-3 (see previous post) and Octocrylene (see previous post).
COMMISSION REGULATION (EU) 2022/1176
On July 7, 2022, the European Commission published the Commission Regulation (EU) 2022/1176, amending Regulation (EC) No 1223/2009 as regards the use of certain UV filter in cosmetic products.
Considering the SCCS opinions on Benzophenone-3 and Octocrylene, the Commission concluded that there is a potential risk to human health arising from the use of these ingredients as UV filters in the concentrations currently allowed and that their use should be restricted to the maximum concentration proposed by the SCCS.
Entries 4 and 10 of Annex VI to Cosmetics Regulation were replaced by the following:
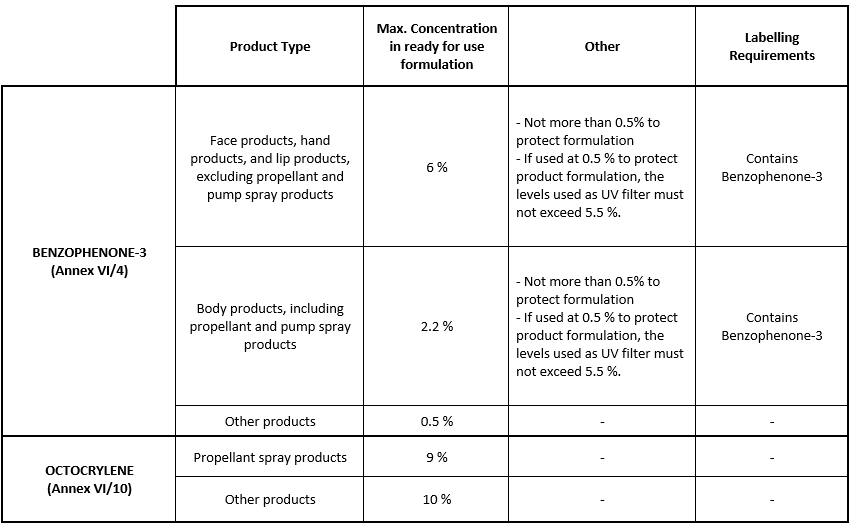
Cosmetic products containing Octocrylene or Benzophenone-3 and complying with the restrictions set out in the European Cosmetics Regulation as applicable on 27 July 2022 may be placed on the market until 28 January 2023 and be made available on the market until 28 July 2023.
If you wish to get more information about this or other subjects, feel free to contact us at info@criticalcatalyst.com.
References:
1. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products.
2. Commission Regulation (EU) 2022/1176 amending Regulation (EC) No 1223/2009 of the European Parliament and of the Council as regards the use of certain UV filters in cosmetic products.
3. Scientific Committee on Consumer Safety (SCCS). Opinion on Octocrylene. SCCS/1627/21. 2021.
4. Scientific Committee on Consumer Safety (SCCS). Opinion on Benzophenone-3. SCCS/1625/20. 2021.















