LEGISLATIVE DECREE Nº 116
The Italian Legislative Decree nº152 of April 2006 has been amended by the Legislative Decree nº 116 of 3 September 2020. This Decree has presented new requirements concerning disposal information that must be reported on the labelling of products (including cosmetic and personal care products, medical devices and food supplements). These obligations apply to packaging material suppliers, but companies must guarantee compliance before making their product available.
The Legislative Decree nº 116 (article 219, paragraph 5) states “all packaging must be appropriately labelled according to the procedures established by the applicable UNI technical standards and in compliance with the decisions adopted by the Commission of the European Union, to facilitate the collection, reuse, recovery and recycling of packaging, as well as to provide consumers with the correct information on their final destinations”. The Decree also refers that all producers have “the obligation to indicate, for the purposes of identification and classification of the packaging, the nature of the packaging’s material used, on the basis of Commission Decision 97/129/EC”.
In sum, this means that it is mandatory to be indicated clearly on the labelling the following information: the nature of the packaging materials used as well as indications concerning the separate collection, reuse, recovery and recycling and correct information to consumers on the final destination of packaging.
Display of information regarding the correct disposal is mandatory on packaging that is provided to the end consumer (B2C circuit), and it is not a requirement when the packaging is destined to “professional” activity (“natural or legal person, or an intermediary thereof, acting in the exercise of his business, commercial, artisanal or professional activity” – B2B circuit) or transport packaging. For B2B packaging (and B2C also) it is mandatory to bear the code of the composition materials (according to Decision 129/97/EC).
The following examples are suggested by CONAI (national packaging private consortium) and the Italian Packaging Institute on their Guidelines on environmental labelling for businesses:
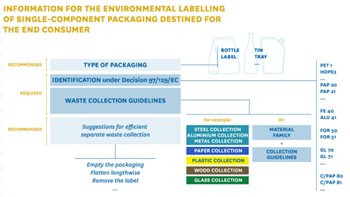
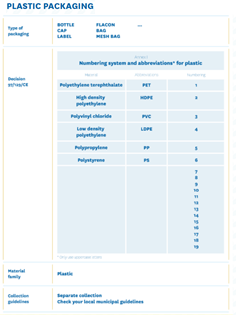
This applies not only for single-component packaging but also for multicomponent packaging (where components can be separated by hand). It is highly recommended that, for multi-component packaging, individual components are identified through a written description or a graphical representation, which will help the consumer to separate and dispose them correctly.
Specific colour codes should be adopted aiming an instant visual recognition on the part of consumers:

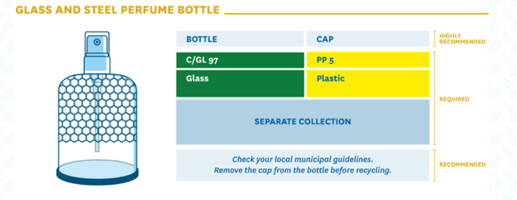
Until December 21st 2021, the requirement for displaying the information indicated in article 216, paragraph 5 (waste disposal), has been suspended by the Decree-Law nº183 (31 December 2020), but some articles of the legislative are already mandatory (e.g. indication of the nature of the packaging material; alphanumerical codes are used to identify the packaging material).
These new Italian environmental requirements may be a major step towards a harmonization of waste disposal labelling in the European Union, alongside with a protection of the environment. Although it is not mandatory for other countries, it can be an advantage for cosmetic companies to adopt these requirements and start adapting their product labels.
References:
- Decreto Legislativo 3 settembre 2020, n. 116. Attuazione della direttiva (UE) 2018/851 che modifica la direttiva 2008/98/CE relativa ai rifiuti e attuazione della direttiva (UE) 2018/852 che modifica la direttiva 1994/62/CE sugli imballaggi e i rifiuti di imballaggio. (20G00135) (GU Serie Generale n.226 del 11-09-2020)
- Decreto Legislativo 3 aprile 2006, n. 152. Norme in materia ambientale. (GU Serie Generale n.88 del 14-04-2006 – Suppl. Ordinario n. 96)
- Decreto-Legge 31 dicembre 2020, n. 183. Disposizioni urgenti in materia di termini legislativi, di realizzazione di collegamenti digitali, di esecuzione della decisione (UE, EURATOM) 2020/2053 del Consiglio, del 14 dicembre 2020, nonche’ in materia di recesso del Regno Unito dall’Unione europea. (20G00206) (GU Serie Generale n.323 del 31-12-2020)
- CONAI and Italian Institute of Packaging Guidelines. Available at: http://www.progettarericiclo.com/en/docs/environmental-labeling-packaging















