INDUSTRIAL CHEMICALS IN AUSTRALIA AND THEIR NEW REGULATION
In Australia, chemicals are regulated depending on their use. If a chemical is not for a therapeutic, agricultural, veterinary or food use, it is considered an industrial chemical. Nearly all cosmetic ingredients are regulated as industrial chemicals under the Industrial Chemicals (Notification and Assessment) Act 1989 (ICNA Act), which is at date administered by NICNAS.
The Industrial Chemicals Act 2019 (IC Act 2019) establishes a new regulatory scheme, called the Australian Industrial Chemicals Introduction Scheme (AICIS), for the importation and manufacture of industrial chemicals in Australia. Chemicals with an industrial use will be now regulated by AICIS.
The framework for managing the transition from the old scheme to the new is set out by the new legislation (the Industrials Chemicals (Consequential Amendments and Transitional Provisions) Act 2019), in addition to IC Act 2019. Three new Industrial Chemicals Charges Acts, authorizing the imposition of charges on importers and manufacturers of industrial chemicals to recover the costs of administering the new scheme, will also take place: Industrial Chemicals Charges (Customs) Act 2019; Industrial Chemicals Charges (Excise) Act 2019; Industrial Chemicals Charges (General) Act 2019.
The Australian Inventory of Chemical Substances (AICS) lists more than 40 000 industrial chemicals that can be manufactured or imported into Australia. AICS contains chemical identity information and regulatory obligations regarding that chemical.
At 30th June 2020, NICNAS website will be archived by the National Library of Australia, but it will remain accessible. A new website web address (AICIS) will be available from July 1st, 2020.
WHAT WILL BE DIFFERENT?
The new scheme (IC Act 2019) implements the ban on the use of new animal test data for ingredients used solely in cosmetics.
After July 1st, 2020, the new inventory will be called Australian Inventory of Industrial Chemicals (AIIC) instead of AICS, and it will be generally referred to as ‘the Inventory’. Although the NICNAS Inventory listed many chemicals that had never had an industrial use, the new Inventory will not list these chemicals.
Regarding chemicals under NICNAS exemptions, companies may introduce these chemicals until 31st August 2022. After this date, companies need to categorize their introduction (explained ahead) to make sure they are compliant with AICIS. The following exemptions are included in this arrangement: R&D (less than 100 kg), no unreasonable risk in cosmetics (less than 100 kg), no unreasonable risk in non-cosmetics (less than 100 kg), and non-hazardous in cosmetics (less than 1%)
Companies which are already registered with NICNAS at 30th June 2020 do not need to do any new registration, as their registration will automatically transfer to AICIS on 1st July 2020, keeping the same registration ID. After 31st August 2020, if companies continue introducing industrial chemicals, they need to renew their registration by 1st September 2020 (online in AICIS Business Services).
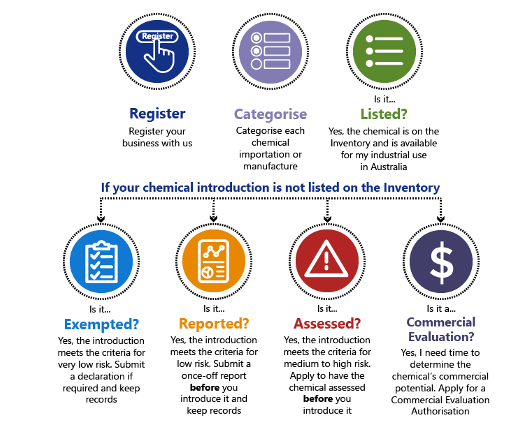
When registering a business, before import or manufacture (introduce) an industrial chemical, each chemical must be authorized under 1 of 5 main categories (called introduction categories). These categories are:
- Listed
- Already on the Inventory and available for industrial use in Australia.
- Exempted
- Considered by AICIS as very low risk to human health and to the environment.
- Reported
- Considered by AICIS as low risk to human health and to the environment
- Assessed
- Considered by AICIS as medium to high risk to human health and the environment.
- Commercial evaluation
- Application for a time-limited Commercial Evaluation Authorisation for the purpose of testing a chemical’s commercial viability in Australia.
Regardless of the introduction category, every introducer must submit an annual declaration at the end of every registration year.
From July 1st 2020, Australian Industrial Chemicals Introduction Scheme (AICIS) will replace the current National Industrial Chemicals Notification and Assessment Scheme (NICNAS), established by a new regulatory scheme (the Industrial Chemicals Act 2019 (IC Act 2019)).
All in all, importers and manufacturers introducing industrial chemicals into Australia for commercial purposes must:
- Register their business and pay a fee (if not already registered with NICNAS).
- Categorize each chemical importation or manufacture (introduction) into 1 of 5 categories.
Importers and manufacturers have also the responsibility to submit declarations and reports, to keep records and give information to AICIS when asked.
References:
- Australian Government, Department of Health – National Industrial Chemicals Notification and Assessment Scheme (NICNAS) – Cosmetics and Therapeutic Goods – https://www.nicnas.gov.au/cosmetics-and-soaps/cosmetics-and-therapeutic-goods
- Australian Industrial Chemicals Introduction Scheme – Fact Sheet – https://www.nicnas.gov.au/__data/assets/pdf_file/0011/94781/AICIS-Fact-Sheet-June-2020.pdf
- Australian Government – Industrial Chemicals (Notification and Assessment) Act 1989 (ICNA Act)
- Australian Government – Industrial Chemicals Act 2019
- Australian Government – Industrial Chemicals (Consequential Amendments and Transitional Provisions) Act 2019
- Australian Industrial Chemicals Introduction Scheme (AICIS) – Industrial Chemicals Categorisation Guidelines – Final Draft, 2019















