ITALY – LEGISLATIVE DECREE Nº 116
The Italian Legislative Decree nº152 of April 2006 has been amended by the Legislative Decree nº 116 of 3 September 2020. This Decree has presented new requirements concerning disposal information that must be reported on the labelling of products (including cosmetic and personal care products, medical devices and food supplements). (further reading in previous publication)
The Legislative Decree nº 116 states that is mandatory to clearly indicate on the labelling the following information:
– The nature of the packaging materials used, indicating the alphanumeric code according to Decision 97/129/EC
- Applicable to packaging intended for professional activity (B2B) and for the end consumer (B2C)
– Indications concerning the separate collection, reuse, recovery and recycling and correct information to consumers on the final destination of packaging.
- Applicable to packaging provided to the end consumer (B2C)
This information must be displayed in Italian.
The Italian Packaging Private Consortium CONAI and the Italian Institute of Packaging have issued Guidelines on environmental labelling. These Guidelines provide some examples of environmental labelling in order to help companies to comply with the Decree.

The Italian environmental labelling obligation is applicable as of January 1, 2023. Packaging already placed on the Italian market or labelled before 1 January 2023 may be marketed until stocks last.
FRANCE – ANTI-WASTE LAW
The French Anti-Waste Law, also known as AGEC law (Loi relative à la lute contre le gaspillage et a l’économie circulaire), contains about 50 measures that include new obligations (requirement of transparency), new prohibitions (control of irreversible ecological ambitions) and new tools to better control and sanction offences against the environment. (further reading in previous publication)
Cosmetic and personal care products that are made available on the French market also need to comply with the French Anti-Waste Law.
The French packaging take-back scheme (CITEO) released a Guideline regarding sorting information aiming to help producers to adjust the packaging labelling in order to be compliant with French requirements.
Since January 2022, the Triman Logo is mandatory and shall be included on the product, its packaging or on the documentation provided with the product. This legal obligation also applies to products sold online to France. It needs to be accompanied by the information on the sorting instructions for each type of product. Any other signs that might confuse consumers regarding the sorting rules should be removed. (further reading in previous publication)
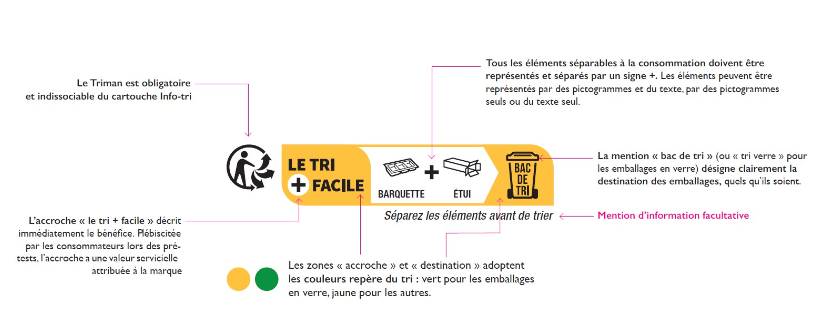
The Triman Logo should be printed in black and white, with a minimum size of 6 mm, and it can not be superimposed by other visual elements or be smaller than symbols printed alongside it. The information regarding the material and the sorting process needs to be in French (including on e-commerce), as this is a specific obligation for the French market.
The French environmental labelling obligation is applicable as of January 1, 2023. A transition period was granted until 9 March 2023, for packaging produced or imported before 9 September 2022.
References:
1. Decreto Legislativo 3 settembre 2020, n. 116. Attuazione della direttiva (UE) 2018/851 che modifica la direttiva 2008/98/CE relativa ai rifiuti e attuazione della direttiva (UE) 2018/852 che modifica la direttiva 1994/62/CE sugli imballaggi e i rifiuti di imballaggio. (20G00135) (GU Serie Generale n.226 del 11-09-2020)
2. CONAI and Italian Institute of Packaging Guidelines. Available at: http://www.progettarericiclo.com/en/docs/environmental-labeling-packaging
3. LOI nº 2020-105 du 10 février 2020 relative à la lutte contre le gaspillage et à l’économie circulaire. Journal Officiel de La République Française. 11 février 2020
4. Ministére de la Tansition Écologique Et Solidaire – The Anti-Waste Law in the Daily Lives of the French People, What Does That Mean in Practice?















