Active devices are classified according to MDR by the classification rules 9, 10, 11, 12 and 13. However, the rules 9 and 10 are applicable only to devices with an intended medical purpose and cannot be applied to active products without a medical purpose, specified in MDR annex XVI. Therefore, such products were classified as class I (according with rule 13) and had to demonstrate conformity with relatively less requirements than analogous devices with a medical purpose.
Certain Member States requested the reclassification of several active products without an intended medical purpose to ensure an appropriate conformity assessment consistent with their inherent risks.
Implementing Regulation (EU) 2022/2347
On 2 December 2022, the Commission published the Implementing Regulation (EU) 2022/2347 in the Official Journal of the European Union, laying down rules for the reclassification of these products.
The following groups of active products without an intended medical purpose listed in MDR Annex XVI are reclassified as follows:
| Section of annex XVI | Product | Previous classification | Updated classification |
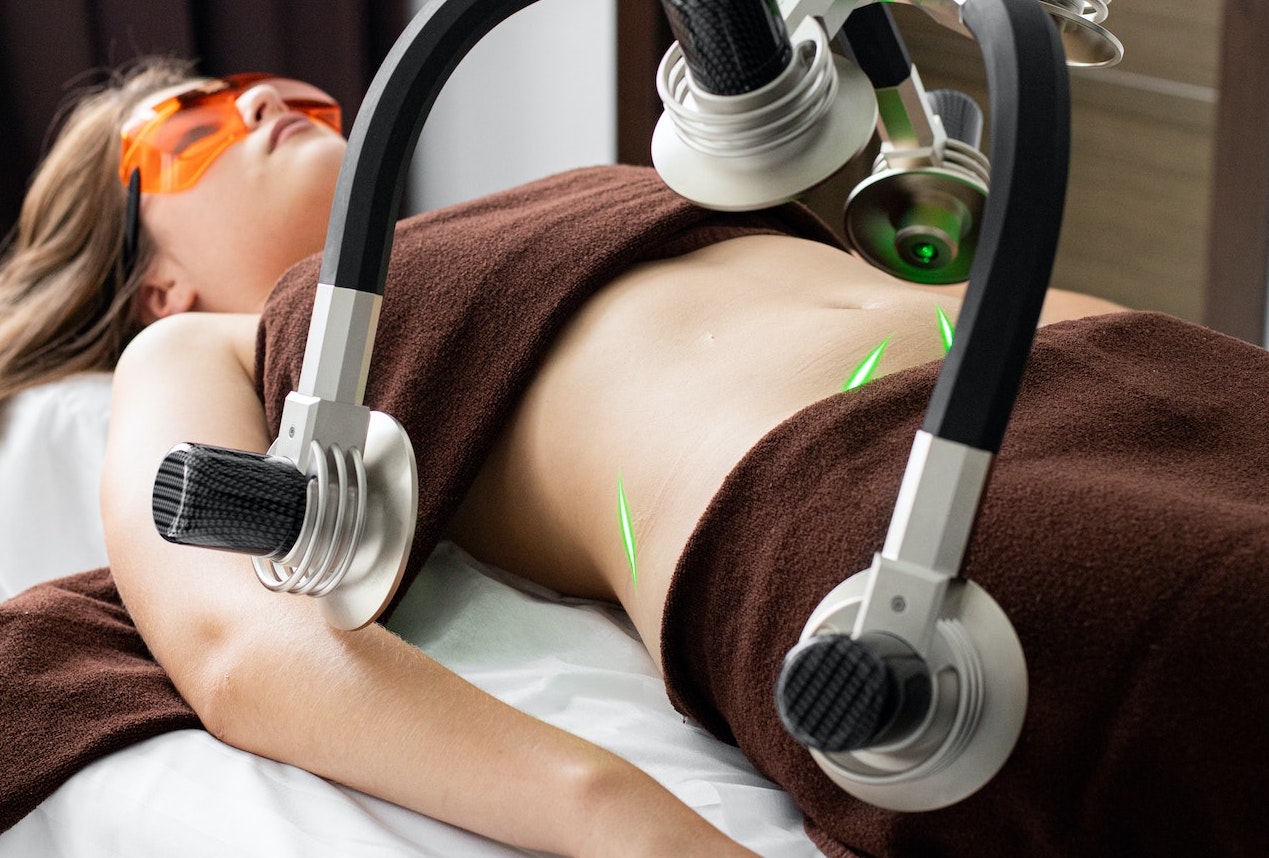
| Section 4 | Equipment intended to be used to reduce, remove or destroy adipose tissue. Ex.: Equipment for liposuction, radiofrequency lipolysis, ultrasound lipolysis, cryolipolyisis, laser lipolysis, infrared and electrical stimulation lipolysis, acoustic shockwave therapy or lipoplasty | Class I | Class IIb |
| Section 5 | Equipment emitting high intensity electromagnetic radiation intended for hair removal. Ex.: Lasers and intense pulsed light equipment | Class I | Class IIa |
| Section 5 | Equipment emitting high intensity electromagnetic radiation intended for skin treatment, skin resurfacing, scar removal, tattoo removal, or for treatment of nevi flammei, haemangioma, telangiectasia and pigmented skin areas. Ex.: Lasers and intense pulsed light equipment | Class I | Class IIb |
| Section 6 | Equipment intended for brain stimulation that apply electrical currents or magnetic or electromagnetic fields that penetrate the cranium to modify neuronal activity in the brain. Ex.: Equipment for transcranial magnetic stimulation or transcranial electric stimulation | Class I | Class III |
The Implementing Regulation (EU) 2022/2347 enters into force on 22 December 2022, is binding in its entirety and directly applicable in all Member States.
For more information feel free to contact us at info@criticalcatalyst.com.
References: