BACKGROUND
Aluminium (Al) and Al compounds are used in a variety of cosmetic products, predominantly in deodorants, antiperspirants, lipsticks, and toothpastes. Several Al compounds are regulated in different entries of the Cosmetics Regulation (EC) No 1223/2009.
In 2013, the SCCS was mandated to evaluate the possible risk for human health arising from the presence of Al in cosmetics. In its opinion in 2014, the Commission concluded that, because of the lack of data on dermal penetration, the risk assessment could not be performed.
After receiving new information regarding dermal penetration, at its plenary meeting on 3-4 March 2020, the SCCS adopted its final Opinion SCCS/1613/19 and in March 2021 an addendum to this Opinion was published. In the addendum, the SCCS concluded that the use of aluminium compounds is safe at the equivalent aluminium concentrations up to:
- 6,25 % in non-spray deodorants or non-spray antiperspirants
- 10,60 % in spray deodorants or spray antiperspirants
- 2,65 % in toothpaste
- 14 % in lipstick.
Following the discussion at the Cosmetics Working Group held on 25 June 2020 and in light of the comments received on the use of Aluminium compounds in a variety of products other than deodorants, antiperspirants, lipsticks and toothpastes, the Commission considered opportune to request from industry to submit additional information on the ‘other product categories’ and on the aggregate exposure not only from cosmetics. In March 2021, industry submitted a dossier focusing on the aggregate exposure to aluminium concerning the European population when considering the use of cosmetics and personal care products, medicines (e.g., antacids) and dietary intake and the SCCS was requested to perform a safety assessment in view of the new information provided.
The current request concerns the update of use concentrations based on submission IV by the Aluminium consortium, which comprises an amendment to the probabilistic exposure assessment report.
WHAT’S NEW?
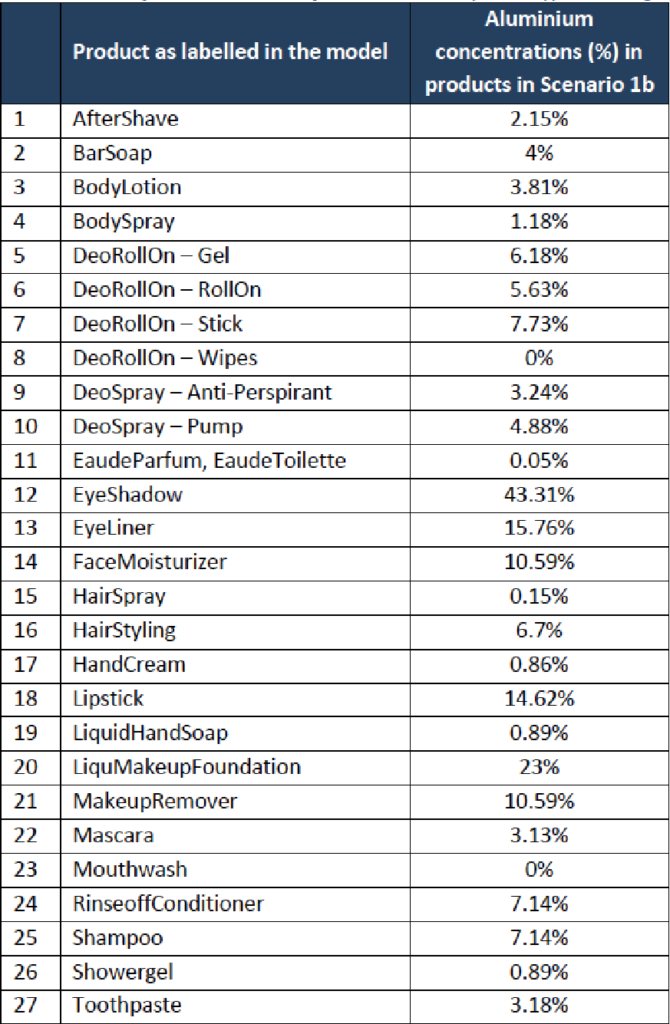
The SCCS considers that aluminium compounds are safe when used:
- in non-sprayable product categories at the maximum levels
- in sprayable products, at the maximum levels for the total formulation (i.e. including propellant), provided that the percentage of particles/droplets with a diameter of less than 10 μm does not exceed 20% of the total aerosolised particles/droplets.
- in talc due to the fact that aluminium is not bioavailable from talc up to a level of 2%
This Opinion does not cover sunscreen aerosol sprays.
As mentioned in Opinion SCCS/1644/22, aluminium does not belong to substances classified as CMR 1A or B, so that only exposure from cosmetic uses was considered in this safety assessment with the exposure assessment based on maximum use levels for cosmetic ingredients. However, as evaluated in SCCS/1644/22, aggregated exposure from cosmetics and food may exceed safe limits for consumers at the highest exposure ranges.
It needs to be noted that this Opinion specifically covers the risk to consumers from exposure to aluminium from cosmetic products. As such, this Opinion does not address the safety of the use of talc in cosmetic products beyond the safety of the aluminium content in talc.
These conclusions are aligned with the ones presented in the preliminary opinion on December 14, 2023.
Additionally, this opinion does not apply to nano forms of aluminium for which a separate specific safety assessment would be needed.
REFERENCES















