On July 7, 2022, the European Commission published the Regulation (EU) 2022/1176, amending the EU Cosmetics Regulation as regards the use of certain UV filter in cosmetic products. The use of Octocrylene and Benzophenone-3 was restricted, following the opinion published by the SCCS published in March 2021.
Considering these SCCS opinion, the European Commission concluded that there is a potential risk to human health arising from the use of these ingredients as UV filters in the concentrations currently allowed and that their use should be restricted to the maximum concentration proposed by the SCCS.
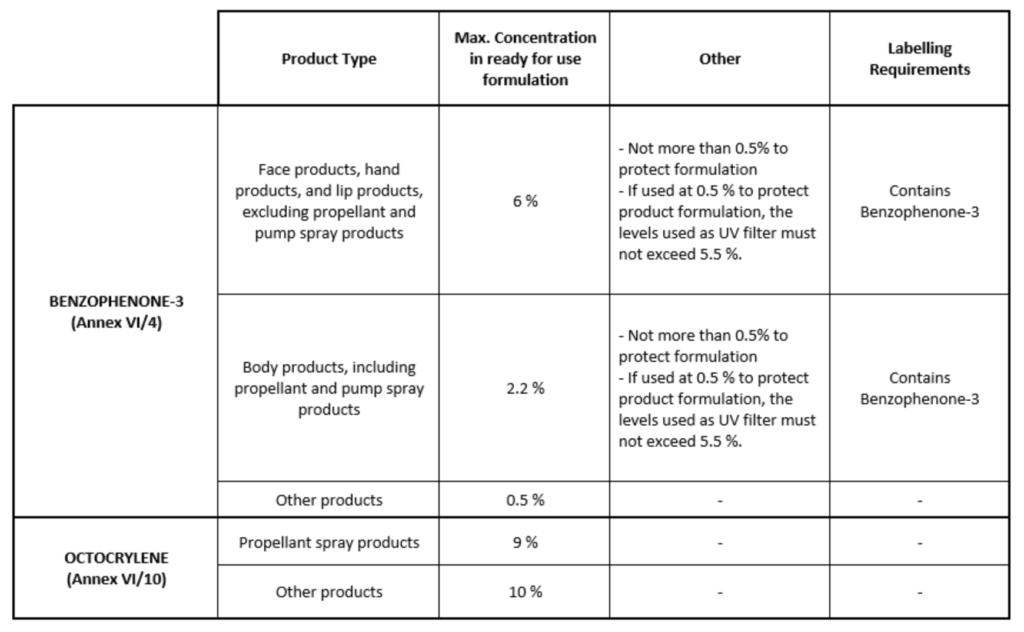
Entries 4 and 10 of Annex VI to Cosmetics Regulation were replaced by the following:
References:
1. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products.
2. Commission Regulation (EU) 2022/1176 amending Regulation (EC) No 1223/2009 of the European Parliament and of the Council as regards the use of certain UV filters in cosmetic products.
3. Scientific Committee on Consumer Safety (SCCS). Opinion on Octocrylene. SCCS/1627/21, 2021.
4. Scientific Committee on Consumer Safety (SCCS). Opinion on Benzophenone-3. SCCS/1625/20, 2021.






