Background
Extended Producer Responsibility (EPR) is a globally implemented policy that motivates producers to design products sustainably, promoting easier reuse and recycling at the end of the product’s life. The UK’s EPR scheme includes a system of packaging producer responsibility, with planned regulatory reforms aimed at improving resource efficiency and reducing waste. The draft specifies the obligations for the producers and sets out detailed requirements for mandatory recycling labels on packaging.
What’s new?
Packaging producers have primary labelling responsibilities according to the notified chapter. These producers must:
- Assess the recyclability of all primary and shipment packaging they supply, according to Regulation 27, and retain the assessment records for at least 7 years.
- Ensure compliance with the recycling labelling requirements for all primary and shipment packaging they supply starting April 1, 2027.
For applicable primary and shipment packaging, it must have a largest surface area of at least 25 cm², excluding exempt packaging specified in Regulation 11(1) and drink containers made of materials other than glass.
The recycling information that must be labelled on the packaging includes a statement and a symbol indicating the packaging’s recyclability, along with relevant recycling instructions. These elements must be displayed together on the packaging in a visible and legible manner. The detailed requirements are presented below:
| Recyclability Assessment Results | Recyclability Information to be Labelled | Symbol | Recycling Instructions* to be Labelled |
| The packaging is not recyclable. | – The phrase “Do Not Recycle” – The following symbol: | 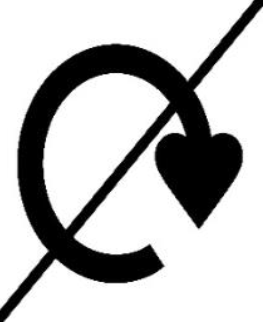 | – |
| The packaging is recyclable. | – The phrase “Recycle” – The following symbol: |  | The instructions on how to dispose of the packaging waste for recycling, other than through household collection by a relevant Authority. |
* The producer shall include recycling instructions on the label if the recyclability assessment has determined that:
– The packaging is not widely collected for household recycling by authorities; and
– Other recycling disposal methods exist for the packaging’s recycling
Other specific requirements can be consulted here.
Special cases:
- Producers supplying medical packaging can include the recycling information on a leaflet inside the packaging or provide it electronically to the intended recipient.
- Producers supplying unbranded filled packaging should either label the recycling information directly on the packaging or on an attached label. For online sales, the recycling information should be displayed in the product description. For offline sales, it must be prominently displayed at the point of purchase.
- When a product’s primary packaging consists of multiple components or ancillary elements, the producer should either label the recycling information on the outer or main component to indicate the recyclability of each part or label each component individually with its recyclability status.
- If a producer becomes aware that any supplied packaging has not been correctly labelled, the producer must take reasonable steps to correct the error.
What now?
The draft is open for comments until June 30, 2024, and is expected to come into force on January 1, 2025.
Once the draft regulation is approved, the producers will be required to assess the recyclability of all primary and shipment packaging they provide. There will be two major dates for labelling requirements:
- 31 March 2026, when the labelling shall be in place for all materials (except plastic films and flexible packaging).
- 31 March 2027, when the labelling shall be in place for plastic films and flexible packaging.







