On 9 June 2023, the European Commission mandated the Scientific Committee on Consumer Safety (SCCS) to evaluate the information submitted regarding Aluminium and review the conclusions of opinions SCCS/1613/19, SCCS/1626/20, and SCCS/1644/22, accordingly. This mandate is based on the European Commission’s commitment to ensuring the safety and appropriate use of Aluminium in cosmetic products. The SCCS approved this mandate during the plenary meeting on 6-7 June 2023, with a deadline of six months for the delivery of its Opinion.
BACKGROUND
Aluminium (Al) and Al compounds are used in a variety of cosmetic products, predominantly in deodorants, antiperspirants, lipsticks, and toothpastes. Several Al compounds are regulated in different entries of the European Cosmetics Regulation No. 1223/2009.
A risk assessment by the Norwegian Scientific Committee for Food Safety in 2013 reported that cosmetic products, and in particular antiperspirants, significantly contribute to the total systemic aluminium exposure, specifically for the Norwegian population.
As a result, SCCS was mandated to evaluate the possible risk for human health arising from the presence of Al in cosmetics. The assessment was based on products and aluminium compounds that contributed to the highest consumer’s exposure, namely antiperspirants/deodorants, toothpastes, and lipsticks. In its Opinion SCCS/1525/142, the SCCS concluded that, due to the lack of adequate data on dermal penetration, the requested risk assessment could not be performed.
However, after industry provided a new safety dossier in 2016, addressing dermal penetration and the fate of Aluminium after skin application, based on a human exposure study, the SCCS adopted its final Opinion SCCS/1613/194 and in March 2021 an addendum to this Opinion was published.
In this addendum, the SCCS concluded that the use of aluminium compounds is safe at the equivalent aluminium concentrations up to:
- 6,25 % in non-spray deodorants or non-spray antiperspirants
- 10,60 % in spray deodorants or spray antiperspirants
- 2,65 % in toothpaste
- 14 % in lipstick
In March 2021, industry submitted a dossier focusing on the aggregate exposure to aluminium
concerning the European population when considering the use of cosmetics and personal care
products, medicines (e.g., antacids) and dietary intake and the SCCS was requested to perform a
safety assessment in view of the new information provided.
In its Opinion SCCS/1644/22, the SCCS concluded that Aluminium compounds are safe when
used in non-sprayable product categories at the maximum levels indicated in Tables 4 and 6 of
the SCCS Opinion as follows:
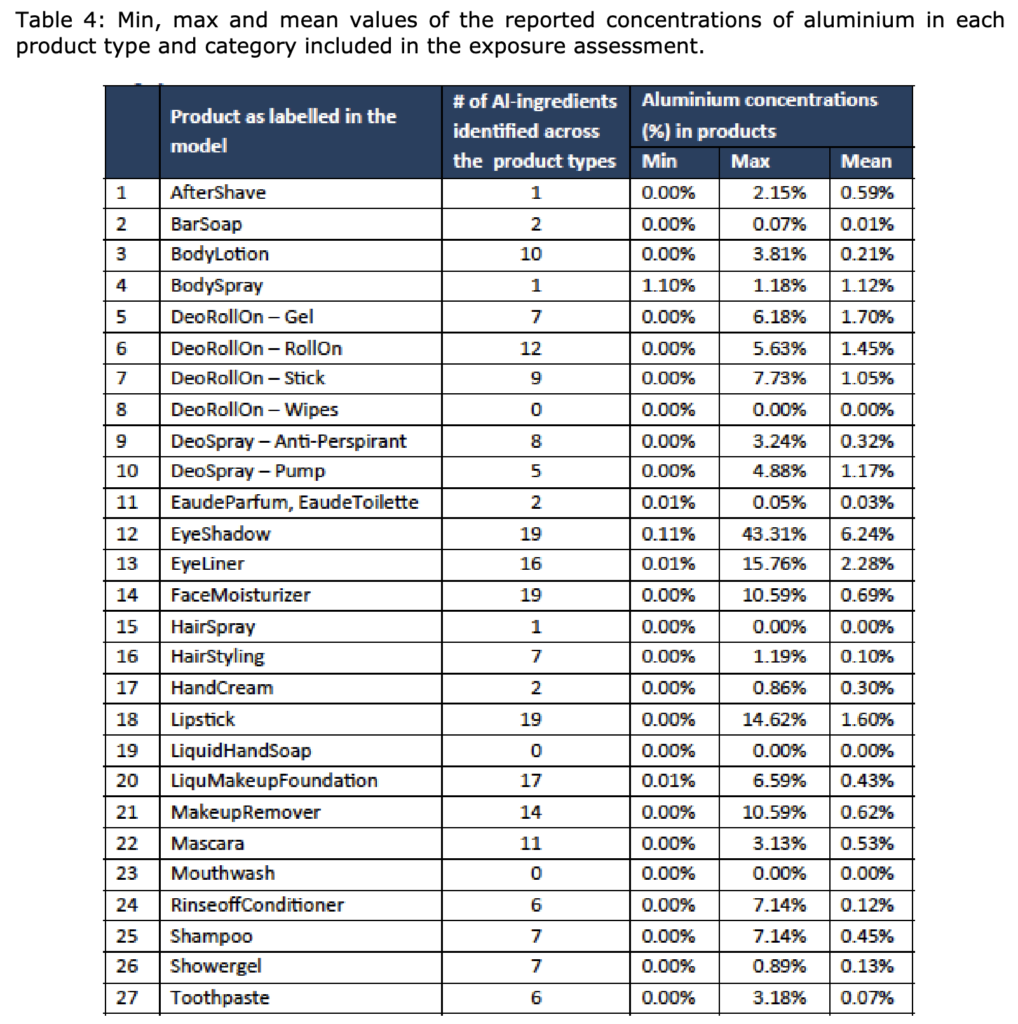
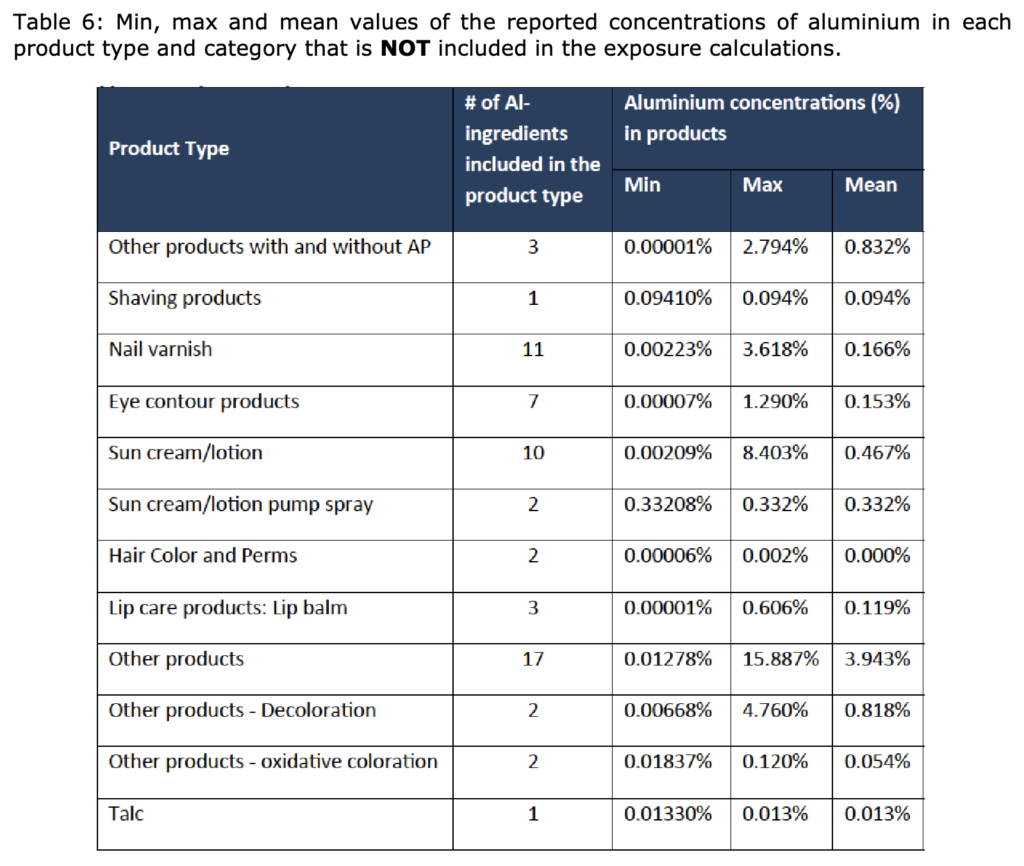
And in sprayable products, at the maximum levels indicated in Table 4, provided that the percentage of particles/droplets with a diameter of less than 10 μm does not exceed 20 % of the total aerosolised particles/droplets. In addition, the SCCS noted that based on a realistic aggregate exposure scenario used in that submission, contribution to Aluminium exposure from food may be at a similar order of magnitude to that from cosmetics.
This information taken together with the conservative nature of the exposure estimates for cosmetics that were used for calculating the Margin of Safety (MoS), the SCCS concluded that the aggregate exposure to aluminium from cosmetic and non-cosmetic sources may exceed safe limits for consumers at the highest exposure ranges.
WHAT’S NEW?
The current request concerns the update of use concentrations based on submission IV by the Aluminium consortium, which comprises an amendment to the probabilistic exposure assessment report. The European Commission requested the SCCS to evaluate the information submitted and review its conclusions in SCCS/1613/19, SCCS/1626/20, and SCCS/1644/22, accordingly.
The SCCS opinion will address the following questions:
- In light of the new data provided, does the SCCS consider Aluminium compounds safe when used in cosmetic products? In the event that the estimated exposure to Aluminium from cosmetic products is found to be of concern, SCCS is asked to recommend safe concentration limits for each category and product type.
- Does the SCCS have any further scientific concerns regarding the use of relevant Aluminium compounds in cosmetic products taking into account the newly submitted information?
References:






