BACKGROUND
Methyl Salicylate (CAS No. 119-36-8) is the ester of methyl alcohol and salicylic acid, used in cosmetics and personal care products as a denaturant, flavouring, oral care, perfuming and soothing agent.
Methyl Salicylate has been classified as toxic for reproduction (CMR substance, category 2) under Regulation (EC) No. 1272/2008 (CLP), but it was not included in the Annexes to Regulation (EC) No. 1223/2009.
WHAT’S NEW?
Following the preliminary opinion on May 16, 2023, the Scientific Committee on Consumer. Safety (SCCS) published its final opinion and scientific advice on children exposure on Methyl Salicylate.
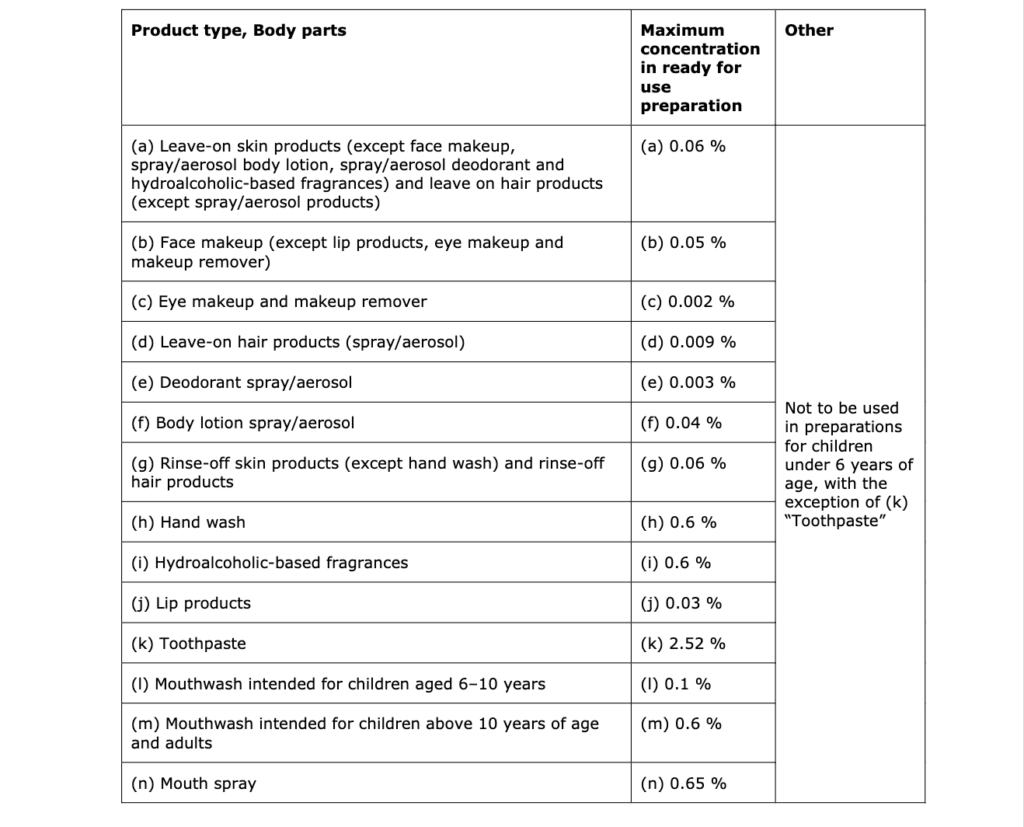
In light of the data provided and taking under consideration the conclusions of SCCS/1633/21 and the aggregate exposure, the SCCS considers the use of Methyl Salicylate as safe in:
- Toothpaste up to a maximum concentration of 2.52%.
- Cosmetic products intended for children of age 0.5-3 years when used up to a maximum concentration of 0.02% in shower gel, hand soap, shampoo, body lotion, face cream, hand cream, lip products and hair conditioner.
- Cosmetic products intended for children of age 3-6 years in shower gel, hand soap, shampoo, body lotion, face cream, hand cream, lip products, and hair conditioner up to the allowed maximum concentrations indicated in the following table:
As no specific data were provided for children below 6 months, the SCCS has not considered this age category in this safety assessment.
References:






