Aluminium (Al) and Al compounds are used in a variety of cosmetic products, predominantly
in deodorants, antiperspirants, lipsticks and toothpastes.
On February 2, 2023, the Scientific Committee on Consumer Safety (SCCS) has published its final opinion on the use of aluminum in cosmetic products. After a commenting period from May 30 to July 2021, the SCCS concludes that aluminum compounds are safe when used within the maximum levels as stated in the opinion.
The SCCS considers that aluminium compounds are safe when used:
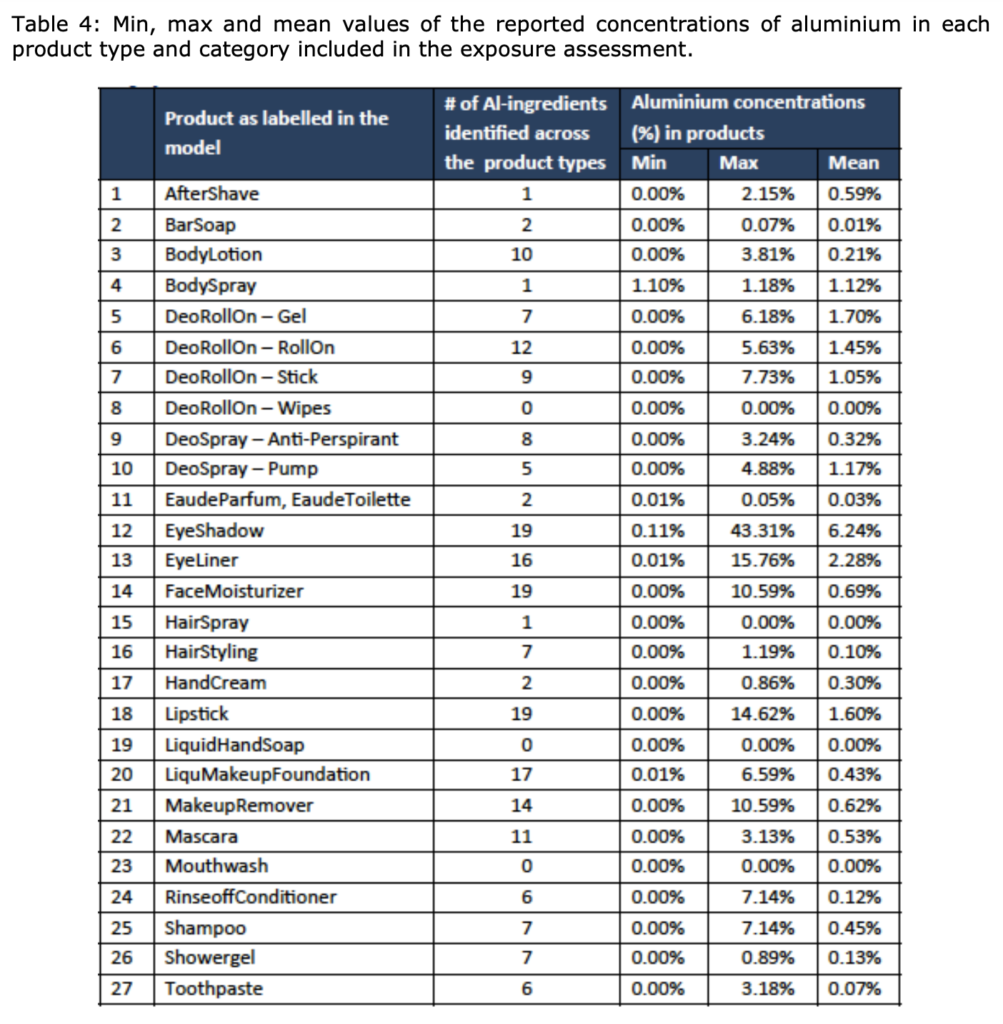
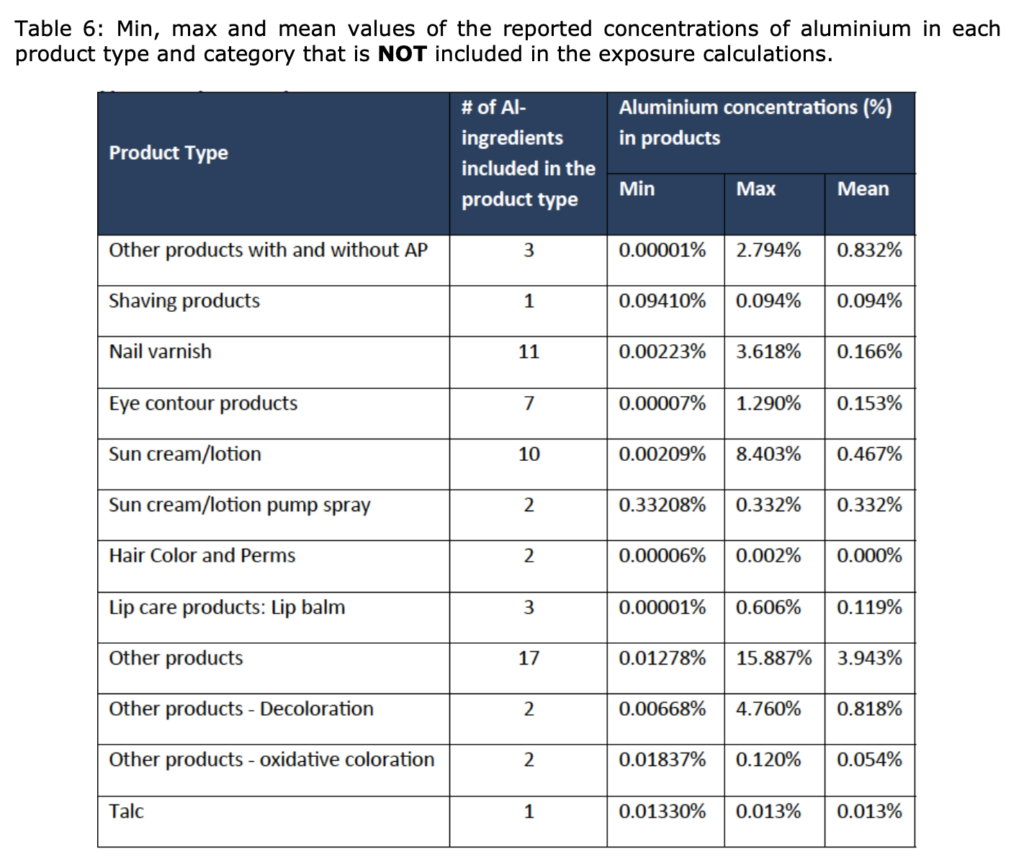
- in non-sprayable product categories at the maximum levels indicated in Tables 4 and 6; and
- in sprayable products, at the maximum levels indicated in Table 4, provided that the percentage of particles/droplets with a diameter of less than 10 μm does not exceed 20% of the total aerosolised particles/droplets. The opinion does not cover sunscreen aerosol sprays.
References:
SCCS (Scientific Committee on Consumer Safety), Opinion on the safety of aluminium in cosmetic products – Submission III, preliminary version of 6 May 2022, final version of 1 February 2023, SCCS/1644/22






