On June 13, 2023, the Scientific Committee on Consumer Safety (SCCS) published the Preliminary Opinion on the use of Benzyl Salicylate in cosmetic products. The opinion is open for comments until August 24, 2023.
BACKGROUND
Benzyl Salicylate (CAS 118-58-1, EC 204-262-9) with the chemical name ‘2- hydroxybenzoic acid phenylmethyl ester’ is produced naturally in a variety of plants and plant extracts where it can be extracted. In addition, Benzyl Salicylate can be synthesised for use, typically as a fragrance/perfuming ingredient, in a range of consumer products, including cosmetics.
Benzyl Salicylate is considered an established contact allergen in humans, following the assessment conclusions of the Scientific Committee on Cosmetic Products and Non-Food Products (SCCNFP) in 1994 and the SCCS in 2012. It is currently regulated for labeling purposes as an allergen in entry 75 of Annex III to the European Cosmetics Regulation No. 1223/2009. In particular, its presence must be indicated in the list of ingredients when its concentration exceeds 0.001% in leave-on products and 0.01% in rinse-off products.
Upon the European Commission’s call for data, stakeholders submitted scientific evidence to demonstrate the safety of Benzyl Salicylate as a fragrance ingredient in cosmetic products. The European Commission requested the SCCS to carry out a safety assessment of Benzyl Salicylate in view of the information provided.
WHAT’S NEW?
In light of the data provided and considering the concerns related to potential endocrine disrupting properties of Benzyl Salicylate, the SCCS considers Benzyl Salicylate safe when used up to the maximum concentrations provided in Table 1 of the Preliminary Opinion, as follows:
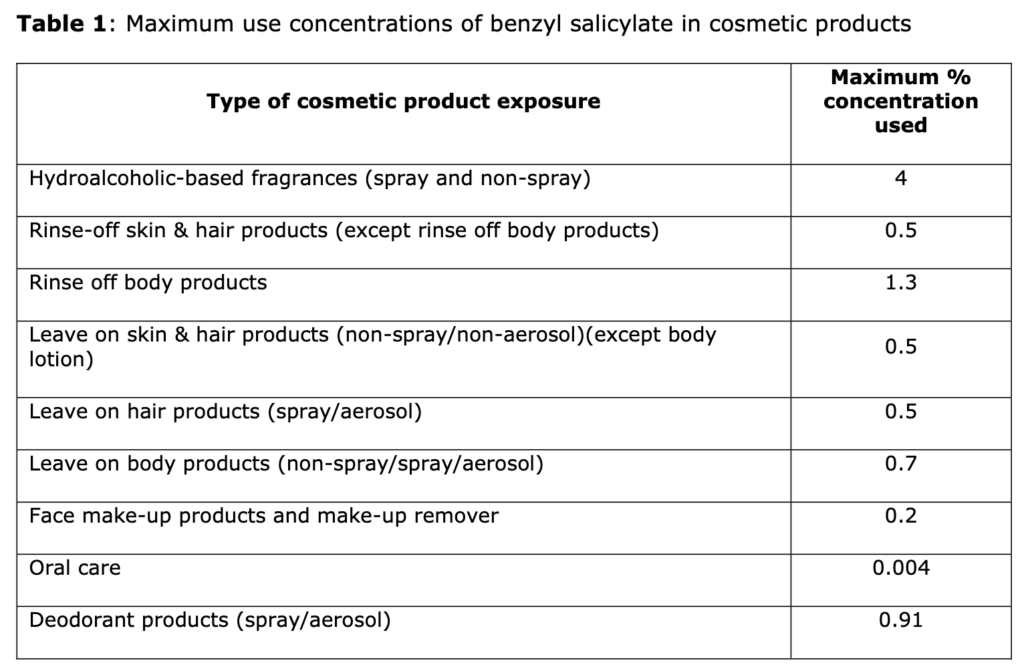
Additionally, the SCCS concluded that the available data on Benzyl Salicylate provide some indications for an endocrine mode of action, but there is no evidence that this results in potential endocrine effects.
References: