On May 16, 2023, the EU Scientific Committee on Consumer Safety (SCCS) published a draft opinion on the use of Methyl Salicylate (CAS No. 119-36-8) in cosmetics intended for children. The draft is open for comments until June 19, 2023.
Background
Methyl Salicylate (CAS number: 119-36-8) is the ester of methyl alcohol and salicylic acid and is used, in cosmetics and personal care products, as a denaturant, flavouring, oral care, perfuming and soothing agent. This substance was classified as toxic for reproduction (CMR substance, category 2) under the CLP Regulation, but it was not included in the Annexes to Regulation (EC) No 1223/2009.
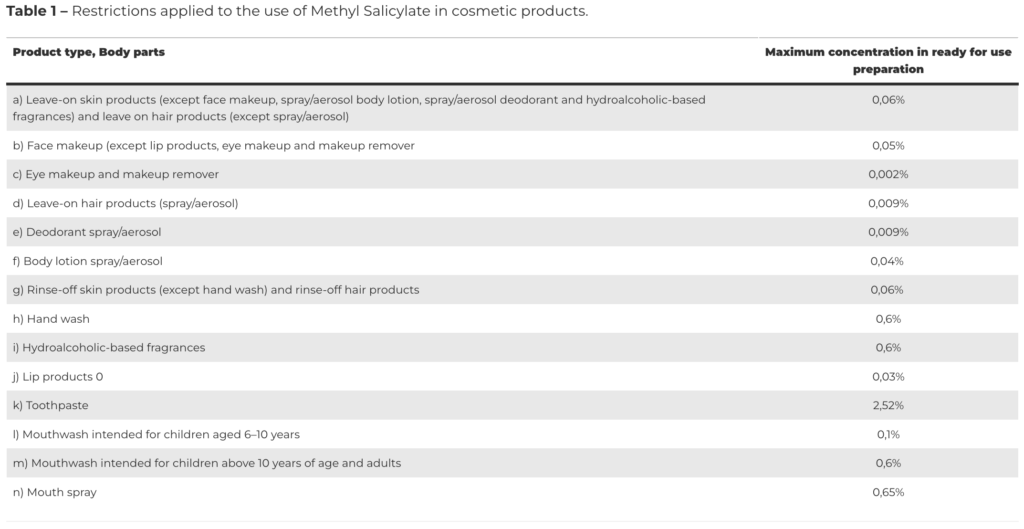
In October 2021, the SCCS issued its final opinion on Methyl Salicylate. The Committee concluded that Methyl Salicylate is considered safe when used in cosmetics up to the maximum concentration provided in the dossier submitted. Considering its classification as a CMR substance of category 2 and the SCCS opinion, the European Commission concluded that Methyl Salicylate should be added to the List of Substances Restricted in Cosmetic Products (Annex III to Regulation, entry 324), in accordance with the following table:
What’s new?
In the SCCS/1633/21 Opinion, the SCCS concluded that Methyl Salicylate in toothpaste is safe for children under 6 years of age when used up to the maximum concentration of 2.52%.
In view of the conclusions of SCCS/1633/21 and the aggregate exposure, the SCCS considers the use of Methyl Salicylate as safe in cosmetic products intended for children of age 0.5-3 years when used up to a maximum concentration of 0.02% in shower gel, hand soap, shampoo, body lotion, face cream, hand cream, lip products. As no specific data were provided for children below 6 months, the SCCS has not considered this age category in this safety assessment.
The preliminary opinion is open for comments until June 19, 2023.
References:
SCCS Scientific Advice – Children exposure on Methyl salicylate (methyl 2-hydroxybenzoate)





