Background
Per- and polyfluoroalkyl substances (PFAS) are a diverse group of approximately 10,000 human-made chemicals which are used in a wide range of consumer and industrial products. Certain PFAS are also intentionally added as ingredients in some cosmetic products, including but not limited to lotions, cleansers, nail polish, shaving cream, foundation, lipstick, eyeliner, eyeshadow, and mascara. These PFAS are used in cosmetics to condition and smooth the skin, making it appear shiny, or to affect product consistency and texture.
Some common PFAS used as ingredients in cosmetics include PTFE (polytetrafluoroethylene), perfluorooctyl triethoxysilane, perfluorononyl dimethicone, perfluorodecalin, and perfluorohexane. Some PFAS may also be present in cosmetics unintentionally as the result of raw material impurities or due to the breakdown of PFAS ingredients that form other types of PFAS.
What’s new?
Because PFAS are very persistent in the environment, the EU authorities estimate that around 4.4 million tonnes of PFAS would end up in the environment over the next 30 years unless action is taken.
The proposal for PFAS restriction was prepared by authorities in Denmark, Germany, the Netherlands, Norway and Sweden and was submitted to the European Chemicals Agency (ECHA).
On February 7, 2023, ECHA announced its proposed restrictions on per- and polyfluoroalkyl substances (PFAS), aiming to reduce PFAS emissions into the environment and make products and processes safer for people as well as supporting the ambitions of the EU’s Chemicals Strategy and the Zero Pollution action plan.
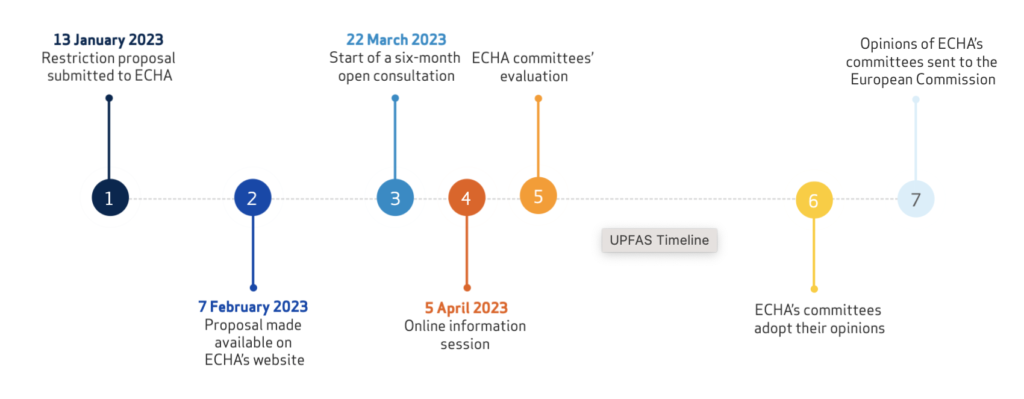
Timeline for next steps
ECHA’s scientific committees for Risk Assessment (RAC) and for Socio-Economic Analysis (SEAC) will check that the proposal meets the legal requirements of REACH Regulation in their meetings in March 2023. If it does, the committees will begin their scientific evaluation of the proposal. A six-month consultation is planned to start on 22 March 2023.
Once the opinions are adopted, they will be sent to the European Commission who, together with the EU Member States, will then decide on the potential restriction (expected 2025). The restriction is expected to enter into force after a transition period of 18 months (2026/2027).
An online information session will be organized on 5 April 2023 to explain the restriction process and to help those interested in participating in the consultation.
References:
1. European Chemicals Agency (ECHA) – PFAS restrictions proposal (ECHA/NR/23/04)







