BACKGROUND
The UK Scientific Advisory Group on Chemical Safety in Consumer Products (SAG-CS) is the independent scientific advisory body to the UK cosmetics regulator, the Office for Product Safety & Standards (OPSS). SAG-CS opinions form the basis for changes to the UK Cosmetics Regulation Annexes for ingredient restrictions.
Methyl Salicylate (CAS No. 119-36-8, EC No. 204-317-7) is a cosmetic ingredient that is frequently part of fragrances used in cosmetic products. It is also used as a flavoring or soothing agent in oral care products. It may be found in hair products, bathing products, perfumery, make-up, and oral hygiene products, amongst others.
The safety of Methyl Salicylate has been evaluated by the EU Scientific Committee on Consumer Safety (SCCS), initially in 2021 and again in 2023, following revised concentrations submitted by industry to support safe use for children.
Methyl Salicylate is not currently listed in the Annexes of the Cosmetic Products Regulation UK (as amended). Under the GB Classification, Labelling and Packaging (CLP) Regulation No 1272/2008 (as amended), Methyl Salicylate has recently been classified as a category 2 reproductive toxicant (suspected of damaging the unborn child), as well as in acute toxicity category 4 and skin sensitiser category 1B.
Article 15 of the UK Cosmetics Regulation prohibits the use of category 1A, 1B and 2 Carcinogenic, Mutagenic or Reprotoxic (CMR) classified substances in cosmetic products under the GB CLP Regulation, unless an exemption has been granted by the Secretary of State following a positive opinion of the SAG-CS.
To obtain an exemption and ensure the continued use of Methyl Salicylate in cosmetic products, the UK Cosmetics Industry have provided a dossier of information and original studies to OPSS, aiming to support the safe use of methyl salicylate in the products listed in the following table:
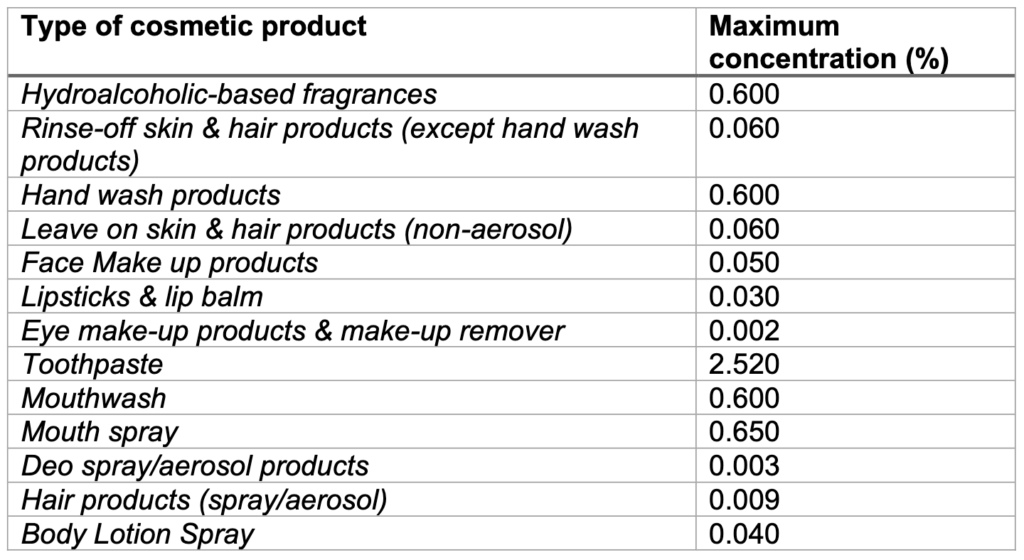
WHAT’S NEW?
On 8 March 2024, the SAG-CS published its final opinion on Methyl Salicylate as used in cosmetics, establishing specific concentration limits for various product types and age groups.
Infants and Toddlers aged 0-3 years: maximum concentration of 0.015% in all dermally apllied products or 2.5% in toothpaste. Hydroalcoholic-based fragrances, face-make up products, eye make-up products and make-up remover, mouthwash and mouth spray have been excluded from the safety assessment for this age group.
Children aged 3-10 years: the current maximum concentrations as in the table above, except in mouthwash where the maximum concentration should be 0.1% (this is a difference when comparing with the EU SCCS opinion). Products not intended for or habitually used by children were excluded, e.g., mouthwash, hydroalcoholic-based fragrances, make-up and deodorant.
Adolescents (10-14 years): the current maximum concentrations as in the table above, except for mouthwash where the maximum concentration of Methyl Salicylate is reduced to 0.4% (this is a difference when comparing with the EU SCCS opinion).
Adults: SAG-CS considered Methyl Salicylate as acceptable for use by adults at the concentrations stated in the table above, even when considering an aggregate of all the product types and exposure routes (dermal, oral and inhalation).
References:







