Background
Hexyl Salicylate (CAS No. 6259-76-3) is an ingredient with sweet, floral, and fruity odour used in formulations of fragrances in multiple consumer goods including cosmetic, household cleaning products,
and detergents.
Currently, Hexyl Salicylate is not listed in the Annexes to the Cosmetic Regulation (EC) No. 1223/2009
and its use is not otherwise restricted in cosmetic products.
The European Risk Assessment Committee (RAC) of the European Chemicals Agency (ECHA) issued in March 2022 an opinion recommending a ‘Toxic for Reproduction Category 2’ (i.e., suspected of damaging the unborn child) and ‘Skin sensitizer Category 1’ classification for Hexyl Salicylate, based on the results of an LLNA assay and on ‘read across’ from the structural analogue Methyl Salicylate and the metabolite Salicylic Acid, respectively.
What’s new?
Following the RAC opinion, it is expected that the European Commission will propose a classification for Hexyl Salicylate as a ‘Toxic for Reproduction Category 2’ and ‘Skin sensitizer Category 1’, updating the Annex VI of CLP Regulation.
However, in December 2022, stakeholders submitted a dossier to support the safe use of Hexyl Salicylate within specific concentration limits for various products types.
On the basis of this dossier, the Commission requests the SCCS to carry out a safety assessment on Hexyl Salicylate in view of the information provided and considering the following questions to be addressed:
1. In light of the data provided and taking under consideration the CMR Cat.2 classification (to be introduced in Annex VI to Reg. 1272/2008), does the SCCS consider Hexyl Salicylate safe when used up to the maximum concentrations provided in the dossier?
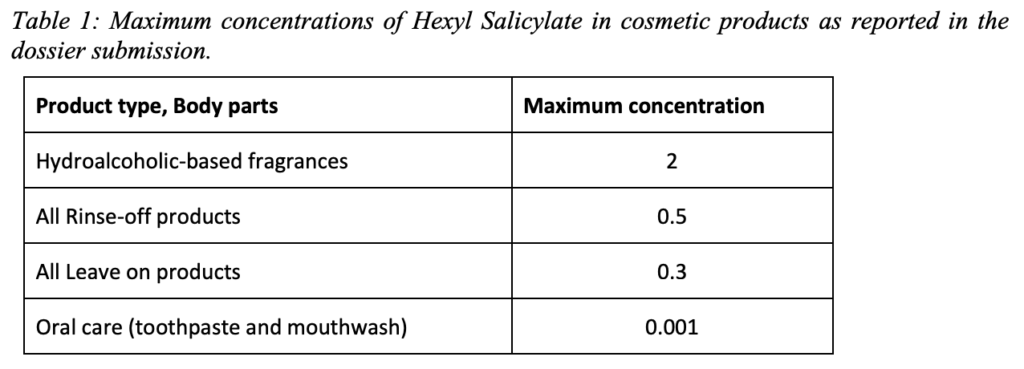

2. Does the SCCS have any further scientific concerns with regard to the use of Hexyl Salicylate in cosmetic products?
The SCCS approved this mandate by written procedure on 13 February 2023, and the Committee has now 9 months to deliver its Opinion.
References:
1. Scientific Committee on Consumer Safety (SCCS) – Mandates






