The International Fragrance Association (IFRA), founded in 1973, represents the interests and is the voice of the fragrance industry worldwide. It promotes the safety and benefits of the fragrance industry’s products through stakeholder dialogue on a global basis.
The IFRA Standards underpin the safe and responsible use of fragrances in consumer products. They are mandatory for IFRA members and are the result of peer reviewed research and safety analysis.
The notification of the 51st Amendment to the IFRA standards was published in 30 June 2023.
WHAT’S NEW?
Relevant updates to the IFRA Standards
- Categorization of pillow spray
- Categorization of reed diffusers
- Handling of traces
- Phototoxicity considerations for products in Category 6
- Elimination of “sunblock” terminology in the guidance
- Clarification that make-up remover for the face and eyes also includes potential exposure to lips
- Clarification about “aftershaves of all types” in Category 4
- Clarification about paper products
- Clarification about the categorization of fabric softener sheets, dryer sheets and dry-cleaning kits
New IFRA Standards
- 1 new IFRA Restriction Specification Standard:
- Allyl Cyclohexylpropionate (CAS 2705-87-5)
- 32 new IFRA Restriction Standards based on dermal sensitization and systemic toxicity:
- 4-tert-Butylcyclohexanone (CAS 98-53-3)
- 1-(2-tert.-Butyl Cyclohexyloxy)-2-Butanol (CAS 139504-68-0)
- Carvomenthone (CAS 499-70-7/ 59471-80-6)
- Citronellyl Acetate (CAS 150-84-5/ 67601-05-2/ 141-11-7)
- Cyclohexadecanone (CAS 2550-52-9)
- Cyclohexadecenone (CAS 88642-03-9/ 5365-06-0/ 2550-59-6/ 3100-36-5/ 5120-20-7/ 854373-71-0/ 854373-70-9
- Alpha-Cyclohexylidene Benzeneacetonitrile (CAS 10461-98-0)
- 2-Cyclohexylidene-2-ortho-tolylacetonitrile (CAS 916887-53-1)
- Dimethyl Octenone (CAS 2550-11-0)
- (Ethoxymethoxy)-cyclododecane (CAS 58567-11-6)
- Ethyl and Methyl Furaneo (CAS 27538-09-6/ 27538-10-9)
- Ethyl Isopropyl Bicycloheptene-2-carboxylate (CAS 116044-44-1/ 116126-82-0)
- cis-3-Heptenyl Acetate (CAS 1576-78-9)
- cis-3-Hexenyl Isovalerate (CAS 35154-45-1)
- cis-3-Hexenyl Methyl Carbonate (CAS 67633-96-9)
- 6-Hydroxy-2,6-dimethylheptanal (CAS 62439-42-3)
- Isobutyl Cinnamate (CAS 122-67-8)
- Isoeugenyl acetate (CAS 93-29-8)
- Isopentylcyclohexanone (CAS 16587-71-6)
- 2-Methyl-2-pentenal (CAS 623-36-9)
- 3-Methyl-5-phenylpent-2-enenitrile (CAS 93893-89-1/ 53243-59-7/ 53243-60-0)
- 4-Methyl-1-propan-2-ylbicyclo[2.2.2]oct-2-ene-8-carboxylate (CAS 68966-86-9)
- Methyl vanillyl ether (CAS 5533-03-9)
- cis-3-Nonenyl acetate (CAS 13049-88-2)
- 3,4,5,6,6-Pentamethylhept-3-en-2-one (CAS 81786-75-6/ 81786-73-4/ 86115-11-9/ 81786-74-5)
- Phenoxyacetaldehyde (CAS 2120-70-9)
- Tetramethyl bicyclo-2-heptene-2-propionaldehyde (CAS 33885-52-8)
- 2,4,4,7-Tetramethyl-6-octen-3-one (CAS 74338-72-0)
- 1-(2,2,6-Trimethylcyclohexyl)-3-hexanol (CAS 70788-30-6)
- 1-(2,2,6-Trimethylcyclohexyl)-3-pentanol (CAS 60241-52-3/ 60241-53-4)
- 3,6,7-Trimethyl-2,6-octadienal (CAS 1891-67-4)
- Woody furan (CAS 338735-71-0, 351343-77-6)
- 11 new IFRA Restriction Standards to control potential dermal sensitization effects solely based on QRA2:
- alpha-Amylcinnamicaldehyde diethyl acetal (CAS 60763-41-9)
- alpha-Amylcinnamicaldehyde dimethyl acetal (CAS 91-87-2)
- Carvyl acetate (CAS 97-42-7/ 1205-42-1/ 1134-95-8)
- 5-Hexen-1-yl 2-methylbutanoate (CAS 155514-23-1)
- 2-Hexylidenecyclohexan-1-one (CAS 16429-07-5)
- Methyl lavender ketone (CAS 67801-33-6/ 67633-95-8)
- Myraldyl acetate (CAS 72403-67-9)
- 2-Octen-4-one (CAS 4643-27-0)
- 3-Octen-2-one (CAS 1669-44-9)
- Octahydro-dimethylnaphthalene-2-carbaldehyde (mixed isomers) (CAS 68738-94-3/ 68738-96-5/ 68991-96-8/ 68991-97-9)
- 2,5-Octadien-4-one, 5,6,7-trimethyl-, (2E)- (CAS 358331-95-0/ 357650-26-1/ 847144-75-6)
- 2 new IFRA Restriction Standards for which risk management is based on Systemic Toxicity (TTC):
- Methoxycyclododecane (CAS 2986-54-1) and
- 7-Methoxy-3,7-dimethyloct-1-ene (CAS 53767-86-5)
- 1 new IFRA Restriction Standard due to potential of depigmentation:
- p-Cresol (CAS 1319-77-3, 106-44-5)
- 1 new IFRA Prohibition Standard due to potential genotoxicity effects:
- 3-Acetyl-2,5-dimethylfuran (CAS 10599-70-9)
Revised IFRA Standards
- 7 Revised IFRA Restriction Standards to control potential dermal sensitization effects for which the systemic toxicity endpoints have now also been evaluated:
- Dihydrocoumarin (CAS 119-84-6)
- Eugenol (CAS 97-53-0)
- Geraniol (CAS 106-24-1)
- 2-Hexenal ( CAS 6728-26-3/ 505-57-7/16635-54-4)
- Hydroxycitronellal (CAS 107-75-5)
- p-Methoxybenzaldehyde (CAS 123-11-5)
- 4-Methoxy-alpha-mehtylbenzenepropanal (CAS 5462-06-6)
- 1 Revised IFRA Restriction Standard based on new dermal sensitization data:
- 3-Propylidenephthalide (CAS 17369-59-4)
- 1 Revised IFRA Restriction Standard based on phototoxicity and systemic toxicity:
- Methyl-N-methylanthranilate (CAS 85-91-6, revised Standard), and
- Tagetes oil and absolute (CAS 91722-29-1/ 8016-84-0/ 91770-75-1)
- 2 Revised IFRA Restriction Standards based on systemic toxicity:
- Estragole (CAS 140-67-0/ 1407-27-8/ 77525-18-9) and
- Methyl Eugenol (CAS 93-15-2)
Timelines
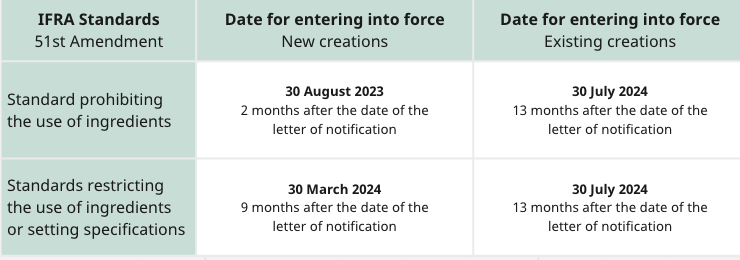
The timelines refer to mixtures of fragrance ingredients and not to finished consumer products.
The date for compliance with the IFRA Amendments corresponds to the date of placement of fragrance mixtures on the market.
“New creations” are defined as any fragrance mixture for which the client’s request has been issued after the completion of the information exchange across the supply chain period.
“Existing creations” are defined as any fragrance mixtures that have already been placed on the market in a consumer product or are already in the development phase at the time the completion of the information exchange comes to its end. This includes:
- fragrance mixtures for which a brief has been received prior to the date of the Notification of
the Amendment; - fragrance mixtures for which the brief has been received during the period of information
exchange across the supply chain; - fragrance mixtures that are already in development by the fragrance manufacturer.
All documentation related to the 51st Amendment is available on the IFRA website via the following links: IFRA Standards documentation (ifrafragrance.org) and IFRA Standards Library (ifrafragrance.org)
References:
The International Fragrance Association (IFRA) – Notification of the 51st Amendment to the IFRA Standards, 30 June 2023






